BELIEVE ME NOT! -  - A SKEPTICs GUIDE
- A SKEPTICs GUIDE


Next: Why Worry, and When?
Up: Radiation Hazards
Previous: Radiation Hazards
One thing we can all agree on is that radiation is
bad for you, right? Well . . . .
First we have to be careful to define what we mean by
``radiation.'' Your fireplace radiates
in the infrared (heat) and visible (light) parts
of the electromagnetic (EM) spectrum; these forms
of radiation are certainly beneficial as long as
they don't get out of control. On the other hand,
visible light in the form of a high-power laser can
inflict damage, as can excessive heat or even
microwave EM radiation.
On the shorter-wavelength side of the EM spectrum,
ultraviolet light can cause sunburn to the skin,
while X-rays penetrate deeper and can do the same
sort of microscopic damage as the still shorter-wavelength
gamma (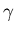 )
rays emitted by 60Co (cobalt)
radioisotopes. Can we make general statements about
all of these? Perhaps, ``A little is good, but
a lot is bad!'' Sorry, nothing so simple.
It is certainly true that we cannot
maintain health without both heat and light,
and a certain amount of ``near ultraviolet''
may be required for natural vitamin D production
in the skin, but we probably have no biological need for
microwave or radio frequency radiation; and
all EM radiation from ``far ultraviolet''
upward in frequency (downward in wavelength)
is exclusively and unambiguously bad
for the individual.2
)
rays emitted by 60Co (cobalt)
radioisotopes. Can we make general statements about
all of these? Perhaps, ``A little is good, but
a lot is bad!'' Sorry, nothing so simple.
It is certainly true that we cannot
maintain health without both heat and light,
and a certain amount of ``near ultraviolet''
may be required for natural vitamin D production
in the skin, but we probably have no biological need for
microwave or radio frequency radiation; and
all EM radiation from ``far ultraviolet''
upward in frequency (downward in wavelength)
is exclusively and unambiguously bad
for the individual.2
Why the big qualitative difference?
What do ultraviolet, X-rays and 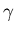 -rays do
that visible and infrared light don't?
At last, a question to which there is a simple answer!
They cause ionization of atoms and molecules
inside cells, leaving behind a variety of free radicals -
types of molecules that quickly react chemically with
other nearby molecules. If the free radicals react with
the DNA molecules in which are encoded
all the instructions to our cells for how to act
and how to reproduce, some of
these instructions can get scrambled.
-rays do
that visible and infrared light don't?
At last, a question to which there is a simple answer!
They cause ionization of atoms and molecules
inside cells, leaving behind a variety of free radicals -
types of molecules that quickly react chemically with
other nearby molecules. If the free radicals react with
the DNA molecules in which are encoded
all the instructions to our cells for how to act
and how to reproduce, some of
these instructions can get scrambled.
Surprisingly, this does not always happen.
The simplest detectable damage to a DNA molecule
is a ``single-strand break,'' in which one of the
strands of the double helix is broken by a chemical
reaction with a radical. It is a testimony to the
robustness of DNA that it is usually able to repair
its own single-strand breaks in a few hours!3
If, however, the DNA molecule
with a single-strand break is subjected to further
damage before it has a chance to ``heal itself''
then it may sustain a ``double-strand break''
(two breaks in the same strand), which it seems
to be far less able to repair. Before we go on to
discuss the consequences of permanent DNA damage,
it is important to note that the irreparable damage
usually takes place only after a large fraction of
DNA molecules have already sustained temporary damage -
and that the temporary damage is mostly repaired in
a fairly short time. This explains why a given
``dose'' of radiation is less harmful when accumulated
over a long time than when delivered in the space of a
few hours.4
What sorts of bad things are liable to happen
when a DNA molecule sustains irreparable damage,
scrambling some part of the instruction manual
for the operation of the cell it inhabits?
- Cell Reproductive Death
[most common] - The cell containing the defective DNA
may be unable to reproduce itself, so that although it may
be able to function normally for its remaining natural
lifetime, when it dies a natural death it will not have
a new cell to replace it. Whether this causes a problem
or not depends upon whether many other nearby cells have
the same malady (one by itself will never be missed!)
and upon the natural lifetime of that type of cell -
which ranges from a few days for hair follicles,
skin and mucous membrane cells
to ``forever'' for brain cells.
Obviously, the loss of reproductive capacity is
meaningless for a cell that never reproduces!
- Genetic Mutation [most subtle] -
If the cell in question happens to be a gamete
destined for fusion with a member of the opposite sex,
the resulting individual will have some scrambled
instructions in the construction manual and will
probably not grow up normally. In almost every case
this will be fatal to the f
 tus, and in almost
all the remaining cases it will be detrimental to
the survival of the individual, although such mutations
have presumably played a rôle in evolution to date.
Note however that it is strictly impossible for
any individual's genetic makeup to be retroactively
altered by radiation (like the Hulk or Spiderman or
any number of cheap sci-fi horrors), as this would require
the same accidental scrambling to take place independently
in every DNA strand in the victim's body!
tus, and in almost
all the remaining cases it will be detrimental to
the survival of the individual, although such mutations
have presumably played a rôle in evolution to date.
Note however that it is strictly impossible for
any individual's genetic makeup to be retroactively
altered by radiation (like the Hulk or Spiderman or
any number of cheap sci-fi horrors), as this would require
the same accidental scrambling to take place independently
in every DNA strand in the victim's body!
For men, there are two types of genetic damage:
the sperm cells themselves have an active lifetime
of only a few days, after which a new generation
takes over; but the sperm-producing cells are never
replaced and so can never repair damage to themselves.
The latter applies also to women: the female gametes
(eggs) are all produced early in life and, once damaged,
cannot be repaired.
If the altered cell is ``just any old cell'' then
usually the change is harmless - either the cell
merely fails to do its part in the body until
it dies or else the affected part of the DNA is irrelevant
to the functioning of that cell in the first place -
but occasionally the change is related to cell division
itself, and then there can be real trouble.
- Cancer [most unpleasant] -
Sometimes (very rarely) a damaged DNA molecule
instructs a cell to mobilize all its resources
and the resources of all its neighbours
to reproduce as many copies of itself as possible.
The offspring preserve the mandate, and a chain reaction
takes place that ``crashes the system.''
This runaway reproductive zeal of a misguided cell
is what we know as CANCER, and it is the worst
hazard of radiation exposure. As far as anyone knows,
any exposure to ionizing radiation increases
one's chances of developing cancer, and so we can
unambiguously say that ionizing radiation
is bad for you.
Before we go on, it is interesting to note that
all of the most potent therapies for
treating cancer involve either
ionizing radiation or chemical reactions that
cause similar DNA damage; the strategy for these
``interventions'' is always to cause such overwhelming
DNA damage to the cancer cells that every single
cancer cell suffers ``cell reproductive death''
as described above. Although there are various
techniques for making the cancer cells more susceptible
to the radiation or harsh chemicals than normal cells,
there are inevitably many casualties among the latter.
It is not unusual, for instance, to kill off (in the sense
of ``reproductive death'') as many as 90% of the normal
cells in the tissues surrounding a tumour, relying upon
the fantastic healing capacity of normal tissue to
bounce back from this insult. Remember, the idea is
to kill 100% (!) of the cancer cells.
It provides an important perspective to realize that
the radiation used to kill the cancer may deliver
a ``dose'' to healthy tissues that is more than 10,000 times
the maximum legal limit for environmental radiation exposure,
and yet the increased likelihood of developing another cancer
from the radiation therapy is regarded as a negligible
risk relative to allowing the existing cancer to progress unchecked.
Whether or not oncologists have optimized their treatment
strategies is another charged issue which I will avoid,
but it is clear that a large radiation dose does not
necessarily ``give you cancer'' immediately; rather
it increases your chances of developing cancer
in the long run. By how much? And over how
long a run? These are the quantitative statistical
questions that must be answered if one is to develop
a rational scheme for evaluating radiation hazards.


Next: Why Worry, and When?
Up: Radiation Hazards
Previous: Radiation Hazards
Jess H. Brewer
1999-11-05
 - A SKEPTICs GUIDE
- A SKEPTICs GUIDE  - A SKEPTICs GUIDE
- A SKEPTICs GUIDE ![]() -rays do
that visible and infrared light don't?
At last, a question to which there is a simple answer!
They cause ionization of atoms and molecules
inside cells, leaving behind a variety of free radicals -
types of molecules that quickly react chemically with
other nearby molecules. If the free radicals react with
the DNA molecules in which are encoded
all the instructions to our cells for how to act
and how to reproduce, some of
these instructions can get scrambled.
-rays do
that visible and infrared light don't?
At last, a question to which there is a simple answer!
They cause ionization of atoms and molecules
inside cells, leaving behind a variety of free radicals -
types of molecules that quickly react chemically with
other nearby molecules. If the free radicals react with
the DNA molecules in which are encoded
all the instructions to our cells for how to act
and how to reproduce, some of
these instructions can get scrambled.