 |
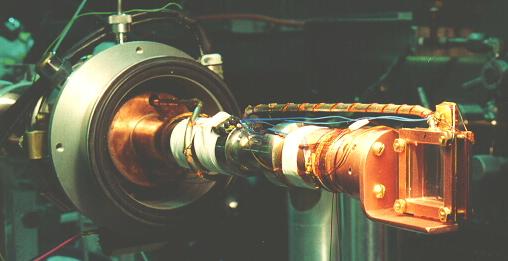
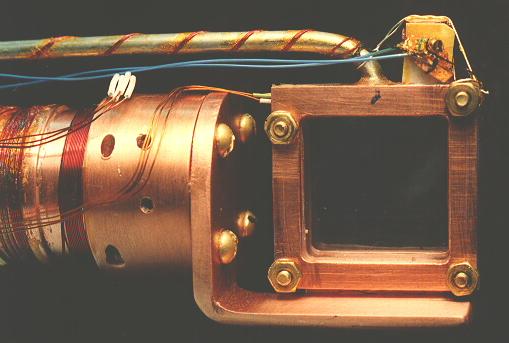
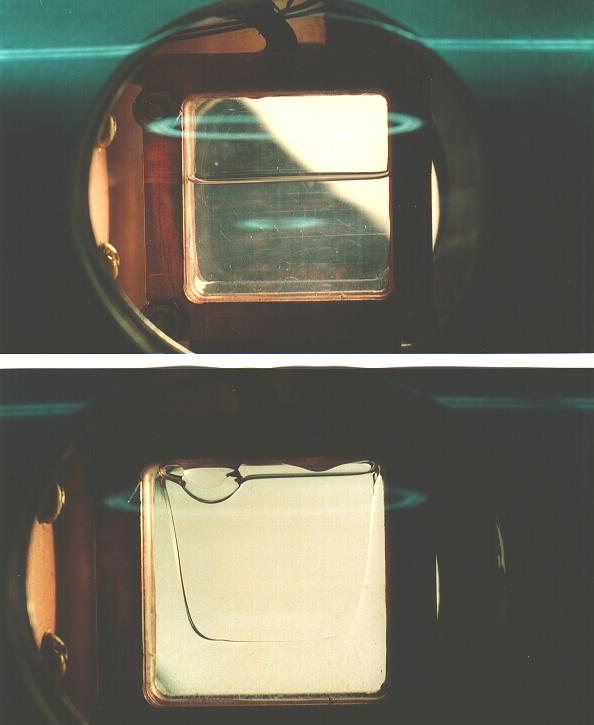
Common experience has shown that the best crystals are obtained by slowly freezing the liquid sample by cooling from the bottom, and allowing the solidifying front to pass upward through the sample. Visual inspection of the sample during its growth is essential, so that the temperature gradient and rate of change of temperature can be controlled by seeing exactly what part of the sample is freezing at any given time.
Samples in this work were always prepared by allowing ultra high purity gas from a clean gas handling system into the cell, which was maintained at a temperature approximately midway between the melting and boiling points. The sample cell was filled slowly, keeping the temperature from rising as the heat of vaporization was taken up. A large amount of heating power, typically 6 W, was applied with the cell top heater in order to establish a temperature gradient down the walls of the cell, enabling one to carefully control the solidification of the sample. By adjustment of the heating and cooling power, the boundary between liquid and solid was made to slowly sweep upward through the sample. After the sample was entirely solid, which typically took 4 hours, the thermal gradient was removed and the sample maintained at a temperature a few degress below the melting temperature for several hours to allow the sample to anneal. Samples so produced were completely transparent, with no visible defects; however, it is known [20] that samples prepared in this way are polycrystalline, with grain dimensions on the order of 1 mm. Very slow crystal growth, much slower than used here, is required to prepare truly single crystals, even of much smaller size. The nature of the crystal structures was not further investigated.
 |
 |
 |